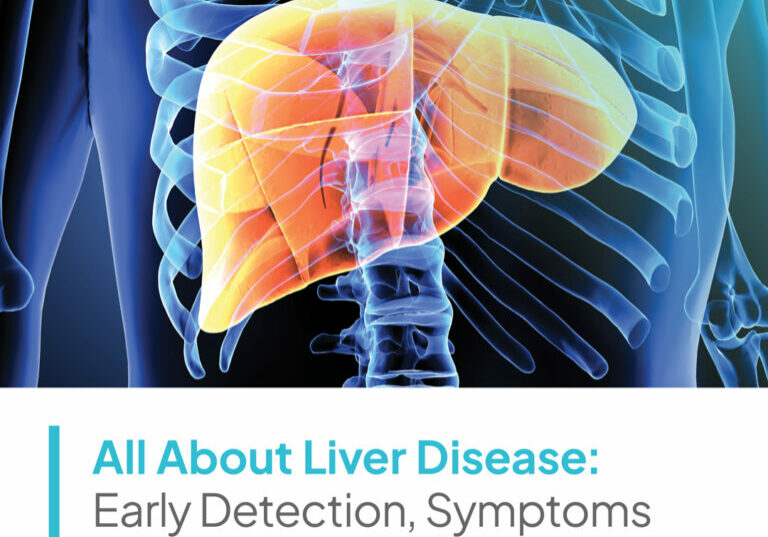
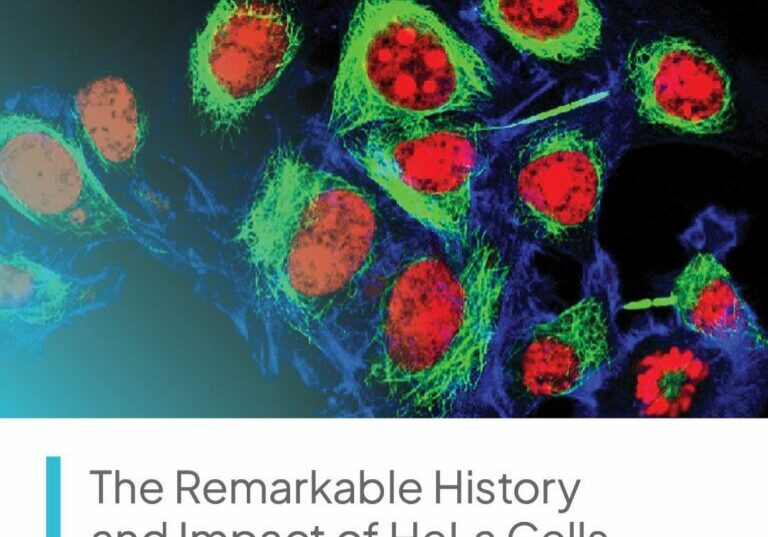
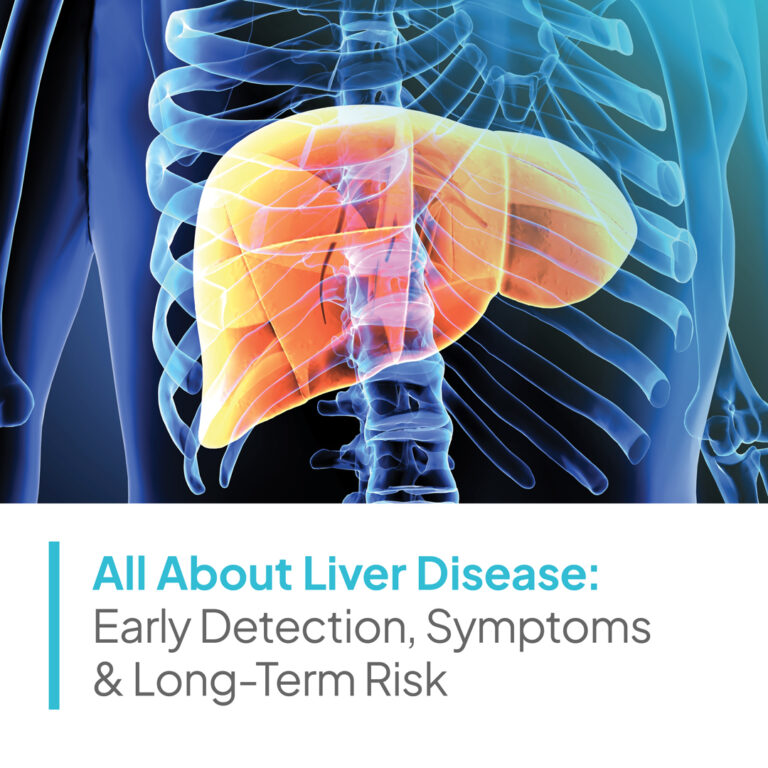
Velocity’s dedicated research site in Washington, DC conducts clinical studies on a multitude of medications and devices intended to address health conditions like acne, allergies, chronic diseases, chronic pain, depression, diabetes, hot flashes, hyperlipidemia, hypertension, infectious diseases, migraine headache, smoking cessation, urinary incontinence, vaccines, viral infections, weight loss, and more.
Located in a populated area near American University and a local hospital, the site is easily accessible to thousands of patients, including a large group of diverse populations. All trials conducted at the site are performed in accordance with ICH and FDA guidelines, and in compliance with GCP. The DC team is committed to being a resource for study participants, to providing the highest quality of patient care with compassion and kindness, and to advancing medicine through research.